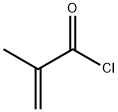
Other grades of this product :
| Methacryloyl chloride Basic information |
| Methacryloyl chloride Chemical Properties |
| Melting point | -60°C | | Boiling point | 95-96 °C(lit.) | | density | 1.08 g/mL at 20 °C | | refractive index | n20/D 1.442(lit.) | | Fp | 57 °F | | storage temp. | 2-8°C | | solubility | Chloroform | | form | Liquid | | color | Clear to slightly colored | | Water Solubility | Miscible with alcohols, ethers and organic solvents. Slightly miscible with water. | | Sensitive | Moisture Sensitive | | BRN | 878175 | | Stability: | Light and Moisture Sensitive | | InChIKey | VHRYZQNGTZXDNX-UHFFFAOYSA-N | | CAS DataBase Reference | 920-46-7(CAS DataBase Reference) | | NIST Chemistry Reference | Methacryloyl chloride(920-46-7) | | EPA Substance Registry System | Methacryloyl chloride (920-46-7) |
| Methacryloyl chloride Usage And Synthesis |
| Chemical Properties | clear slightly colored liquid | | Uses | Methacryloyl chloride is used in the preparation of polymeric materials. It reacts with L-histidine and forms functional monomer. It is also used to prepare monomer 2-methacrylamido-caprolactam, amide monomers by amidation. | | Application | Methacryloyl chloride is used in the manufacture of polymers:Monomer 2-methacrylamido-caprolactam was prepared by reacting methacryloyl chloride with racemic a-amino-e-caprolactam.Functional monomer was prepared by reacting methacryloyl chloride and L-histidine.A series of amide monomers were synthesized by amidation of methacryloyl chloride with amines and grafted onto commercial poly(ether sulfone) (PES) membranes using irradiation from atmospheric pressure plasma (APP).Reaction of methacryloyl chloride with the hydroxyl groups on the surfaces of HEMA/NVP microspheres was performed, leading to the introduction of polymerisable double bonds onto the surfaces of microspheres.Star-shaped poly(d,l-lactide) oligomers with 2, 3 and 6 arms were synthesised, end-functionalised with methacryloyl chloride and photo-crosslinked in the presence of ethyl lactate as a non-reactive diluent. | | General Description | Methacryloyl chloride is the clear to slightly colored acid chloride of methacrylic acid. It appears as a liquid of density 1.07g/cm3, boiling point 95-96°C, and flash point 55°F. Highly toxic. Supplied in technical grades of varying purity. Monomethyl ether hydroquinone is added as a stabilizer to prevent auto polymerization. Reaction with diisopropyl ether in presence of metal salts is rigorous. | | Air & Water Reactions | Highly flammable. Reacts with water to produce gaseous hydrogen chloride (corrosive and toxic). | | Reactivity Profile | Methacryloyl chloride is incompatible with strong oxidizing agents, alcohols, bases (including amines). Can polymerize violently; but the polymerization reaction can be inhibited by the addition of phenolthiazine. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. | | Hazard | Flammable and corrosive liquid. | | Purification Methods | Purify the ester by fractional distillation. If it contains the acid (OH bands in the IR) then add redistilled SOCl2 (with cooling) and cuprous chloride (ca to 2%), reflux the mixture gently for 1hour and fractionate it through a 1metre column packed with glass helices. Redistillation then provides the acid chloride in high purity as a colourless liquid. It is necessary to keep the apparatus moisture free (use CaCl2 tubes), Stabilise it with 0.05% of 2,6-di-tert-butyl-4-methylphenol. [Lal & Green J Org Chem 20 1032 1955, Beilstein 2 IV 1537.] |
| Methacryloyl chloride Preparation Products And Raw materials |
|