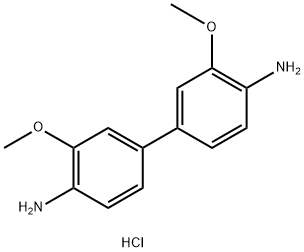
| Description | o-Dianisidine (dihydrochloride) is a colorimetric peroxidase substrate that has been used as a substrate to semi-quantitatively measure lactate, uric acid , and glucose. It has also been used as a reagent for the detection of various metals, thiocyanates, and nitrites. This aromatic amine is initially colorless but turns to violet upon oxidization and is spectrophotometrically measured at a wavelength of 405 nm. |
| Chemical Properties | Off-white or grey tablets |
| Uses | o-Dianisidine (3,3′-dimethoxybenzidine) has been used as a component of GO (glucose oxidase) reagent, which is used for measuring the glucose content. It has also been used as a substrate to measure myeloperoxidase activity. |
| Uses | It is suitable for use in ELISA procedures. In neurohistochemistry o-Dianisidine dihydrochloride has demonstrated perikaryal labeling as well as retrograde and anterograde transport to the primary olfactory cortex. It has also been used for a paper based-micro fluidic device to semi-quantitatively measure lactate, uric acid, and glucose. 3,3'-Dimethoxybenzidine is used principally as an intermediate in the production of commercial bisazobiphenyl dyes for coloring textiles, paper, plastic, rubber, and leather. |
| General Description | Off-white powder. |
| Air & Water Reactions | Water soluble. |
| Reactivity Profile | 3,3'-Dimethoxybenzidine dihydrochloride is an ether substituted acidic organic salt. Reacts weakly as a acid to neutralize bases. May react with strong oxidizing agents or strong reducing agents. |
| Fire Hazard | Flash point data are not available for 3,3'-Dimethoxybenzidine dihydrochloride, but 3,3'-Dimethoxybenzidine dihydrochloride is probably combustible. |
| Safety Profile | Confirmed carcinogen
with experimental carcinogenic and
tumorigenic data. Experimental
reproductive data. Mutation data reported.When heated to decomposition it emits very
toxic fumes of NO, and HCl. |