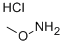Other grades of this product :
| Methoxyammonium chloride Chemical Properties |
| Melting point | 151-154 °C(lit.) | | Boiling point | 105-110°C | | density | 1.1 g/mL at 25 °C | | refractive index | n20/D 1.4021 | | storage temp. | Sealed in dry,Room Temperature | | solubility | alcohol: soluble(lit.) | | form | Crystalline Powder | | color | White to very faint yellow | | Water Solubility | SOLUBLE | | Sensitive | Hygroscopic | | Merck | 14,5989 | | BRN | 3589723 | | InChIKey | XNXVOSBNFZWHBV-UHFFFAOYSA-N | | CAS DataBase Reference | 593-56-6(CAS DataBase Reference) | | EPA Substance Registry System | O-Methylhydroxylamine hydrochloride (593-56-6) |
| Methoxyammonium chloride Usage And Synthesis |
| Methoxyamine hydrochloride | Methoxyamine hydrochloride (also known as O-methylhydroxylamine hydrochloride; Methoxyamine; Methoxyamine hydrochloride (OMHA), is an important medicine, pesticide intermediates. Its major applications are as follows:
- it can be used for color photography and film printing.
- it is used as a reducing agent in organic synthesis industry for preparation of oximes.
- the field of medicine: it can be used for the synthesis of second-generation cephalosporins antibiotics cefuroxime (ester), neopropene, norethindine and hydroxyurea.
- the field of pesticides: the synthesis of new efficient, low-toxicity bactericidal metominostrobin.
- It is mainly produced by sodium nitrite method in our country.
The similar compound of methoxyamine hydrochloride, the ethoxylamine hydrochloride is mainly used for the production of herbicides, oxycarbazone, diltiazem and other herbicides. | | Application |
- Used as a methoxyamine reagent, also used in the production of the side chain of Cefuroxime and other new drugs
- Used for pesticide synthesis.
- Use for pharmaceuticals production
- Used for the preparation of O-methyloxime together with aldehydes or ketones.
| | Chemical Properties | White to very faintly yellow crystalline powder | | Uses | antineoplastic, hydroxymethyltransferase inhibitor | | Uses | Methoxylamine is a methoxime derivative used as internal standard for Prostaglandin assays by gas chromatography-mass spectrometry | | Uses | Methoxylamine hydrochloride is used as a reagent in the preparation of O-methyl oximes from aldehydes. It is orally bioavailable small molecule inhibitor with potential adjuvant activity. It is also used as internal standard for prostaglandin assays by gas chromatography-mass spectrometry. | | General Description | Off-white crystals. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | In aqueous solution, Methoxyammonium chloride behaves as an acid. Materials in this group are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. | | Fire Hazard | Flash point data for Methoxyammonium chloride are not available. Methoxyammonium chloride is probably combustible. | | Purification Methods | Crystallise the hydrochloride from absolute EtOH or EtOH by addition of diethyl ether. [Kovach et al. J Am Chem Soc 107 7360 1985, Beilstein 1 IV 1252.] |
| Methoxyammonium chloride Preparation Products And Raw materials |
|