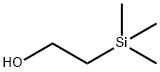
| 2-(Trimethylsilyl)ethanol Basic information |
| Product Name: | 2-(Trimethylsilyl)ethanol |
| Synonyms: | 2-HYDROXYETHYLTRIMETHYLSILANE;2-(TRIMETHYLSILYL)ETHANOL;2-(TRIMETHYLSILYL)ETHANAL;2-(trimethylsilyl)-ethano;TIMTEC-BB SBB009030;2-(TRIMETHYLSILYL)ETHANOL, 97%2-(TRIMETHYLSILYL)ETHANOL, 97%2-(TRIMETHYLSILYL)ETHANOL, 97%;Ethanol, 2-(trimethylsilyl)-;Silane, (2-hydroxyethyl)trimethyl- |
| CAS: | 2916-68-9 |
| MF: | C5H14OSi |
| MW: | 118.25 |
| EINECS: | 220-844-5 |
| Product Categories: | Si (Classes of Silicon Compounds);Si-(C)4 Compounds;Alcohols and Derivatives;Silicon Compounds (for Synthesis);Synthetic Organic Chemistry;bc0001 |
| Mol File: | 2916-68-9.mol |
| 2-(Trimethylsilyl)ethanol Chemical Properties |
| Boiling point | 71-73 °C35 mm Hg(lit.) |
| density | 0.825 g/mL at 25 °C(lit.) |
| refractive index | n |
| Fp | 123 °F |
| storage temp. | Sealed in dry,2-8°C |
| form | Liquid |
| pka | 15.38±0.10(Predicted) |
| color | Clear colorless |
| Specific Gravity | 0.825 |
| Water Solubility | soluble |
| Hydrolytic Sensitivity | 4: no reaction with water under neutral conditions |
| BRN | 1732034 |
| InChIKey | ZNGINKJHQQQORD-UHFFFAOYSA-N |
| CAS DataBase Reference | 2916-68-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Trimethyl-2-hydroxyethylsilane(2916-68-9) |
| Safety Information |
| Hazard Codes | Xi,F |
| Risk Statements | 10-36/37/38 |
| Safety Statements | 16-37/39-26-24/25 |
| RIDADR | UN 1987 3/PG 3 |
| WGK Germany | 3 |
| RTECS | KM5480000 |
| Hazard Note | Flammable |
| TSCA | No |
| HazardClass | 3 |
| PackingGroup | III |
| HS Code | 29310095 |
| MSDS Information |
| Provider | Language |
|---|---|
| 2-(Trimethylsilyl)ethanol | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| 2-(Trimethylsilyl)ethanol Usage And Synthesis |
| Chemical Properties | CLEAR COLOURLESS LIQUID |
| Physical properties | bp 50–52 °C/10 mmHg, 71–73 °C/35 mmHg; d 0.825 g cm?3. |
| Uses | 2-(Trimethylsilyl)ethanol is used as a protecting reagent for carboxyl, phosphoryl, hydroxyl and amino group in organic synthesis. It is used as a precursor to prepare trimethyl(2-phenoxyethyl)silanes by reacting with aromatic fluoride. It is also used in the synthesis of teoc-protected amines by using the corresponding isocyanates. |
| Uses | Used to synthesize Teoc-protected amines via alcoholysis of the corresponding isocyanates. |
| Uses | 2-(Trimethylsilyl)ethanol is a protecting reagent for carboxyl, phosphoryl, hydroxyl, and amino groups. It participates in the reactions of Phenol and Acid Protection, Alcohol Protection, Hemiacetal Protection, Amine Protection, Enol Ether Synthesis, Carbohydrate Chemistry etc. |
| Preparation | Three methods of preparation have been reported: (a) from the treatment of ethyl bromoacetate with zinc followed by the reaction with chlorotrimethylsilane1 and subsequent reduction of the resultant ethyl trimethylsilylacetate with lithium aluminum hydride2,3 or borane–tetrahydrofuran(eq 1); (b) from the hydroboration/oxidation or oxymercuration/demercuration of vinyltrimethylsilane (eq 2); and (c)most conveniently, by the reaction of the Grignard reagent formed from (chloromethyl)trimethylsilane with paraformaldehyde (eq 3). |
| Purification Methods | If the NMR spectrum is not clean, then dissolve the alcohol in Et2O, wash it with aqueous NH4Cl solution, dry (Na2SO4), evaporate and distil it. The 3,4-dinitrobenzoyl derivative has m 66o (from EtOH). [NMR: Speier et al. J Am Chem Soc 79 974 1957, Z Naturforsch 14b 137 1959, Beilstein 4 IV 3951.] |
| 2-(Trimethylsilyl)ethanol Preparation Products And Raw materials |
| Preparation Products | 1-(2-(TRIMETHYLSILYL)ETHOXYCARBONYLOXY)& |
Please leave a message for us or use the following ways to contact us, we will reply to you as soon as possible, and provide you with the most sincere service, thank you.