Other grades of this product :
| 1,1,1-Trifluoroacetone Basic information |
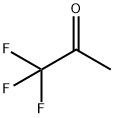
| Product Name: | 1,1,1-Trifluoroacetone | | Synonyms: | TRIFLUOROACETONE;1,1,1-trifluoro-2-propanon;3,3,3-Trifluoroacetone;CH3COCF3;Methyl trifluoromethyl ketone;Trifluoromethyl methyl ketone;1,1,1-TRIFLUOROACETONE;1,1,1-TRIFLUORO-2-PROPANONE | | CAS: | 421-50-1 | | MF: | C3H3F3O | | MW: | 112.05 | | EINECS: | 207-005-9 | | Product Categories: | C3 to C6;Carbonyl Compounds;Ketones | | Mol File: | 421-50-1.mol |
| 1,1,1-Trifluoroacetone Chemical Properties |
| 1,1,1-Trifluoroacetone Usage And Synthesis |
| Chemical Properties | 1,1,1-Trifluoroacetone is CLEAR COLOURLESS LIQUID
| | Uses | 1,1,1-Trifluoroacetone is used as intermediate of bioactive substance and antihypertensive drug, and as effective synthetic building block and trifluoromethyl reaction reagent.
| | Uses | 1,1,1-Trifluoroacetone is a general chemical reagent used in the synthesis of glucokinase-glucokinase regulatory protein) GK-GKRP disruptors which may act as potential targets for type II diabetics. Reactant for enantioselective cycloadditions. | | Uses | Used in a synthesis of 2-trifluoromethyl-7-azaindoles starting with 2,6-dihalopyridines. The derived chiral imine was used to prepare enantiopure α-trifluoromethyl alanines and diamines via a Strecker reaction followed by either nitrile hydrolysis or reduction. |
| 1,1,1-Trifluoroacetone Preparation Products And Raw materials |
|