Other grades of this product :
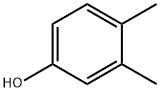
| Product Name: | 3,4-Dimethylphenol | | Synonyms: | 1,2-Dimethyl-4-hydroxybenzene;1,3,4-Xylenol;3,4-dimethyl-pheno;4,5-Dimethylphenol;3 4-XYLENOL 98+%;3,4-DIMETHYLPHENOL PESTANAL;3,4-Dimethylphenol, 98+%;3, 4-Xylenol (3,4-Dimethylphenol) | | CAS: | 95-65-8 | | MF: | C8H10O | | MW: | 122.16 | | EINECS: | 202-439-5 | | Product Categories: | Aromatics;Aromatic Phenols;Industrial/Fine Chemicals | | Mol File: | 95-65-8.mol |
| 3,4-Dimethylphenol Chemical Properties |
| Melting point | 65-68 °C | | Boiling point | 227 °C(lit.) | | density | 1,138 g/cm3 | | vapor pressure | 0.475-130Pa at 25-66.2℃ | | FEMA | 3596 | 3,4-XYLENOL | | refractive index | 1.5442 | | Fp | 61 °C | | storage temp. | Store below +30°C. | | solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) | | pka | pK1:10.32 (25°C) | | form | Crystalline Powder | | color | Off-white to pale cream | | explosive limit | 1.4%(V) | | Water Solubility | SLIGHTLY SOLUBLE | | Merck | 14,10082 | | JECFA Number | 708 | | BRN | 1099267 | | Exposure limits | ACGIH: TWA 1 ppm | | InChIKey | YCOXTKKNXUZSKD-UHFFFAOYSA-N | | LogP | 2.23 at 25℃ | | CAS DataBase Reference | 95-65-8(CAS DataBase Reference) | | NIST Chemistry Reference | Phenol, 3,4-dimethyl-(95-65-8) | | EPA Substance Registry System | 3,4-Dimethylphenol (95-65-8) |
| 3,4-Dimethylphenol Usage And Synthesis |
| Description | 3,4-methylphenol (also 3,4-xylenol) has a flat, dry odor. It can be used as a flavoring agent without safety concern at current levels of intake. 3,4-dimethylphenol is used as an agent in the spectrophotometric method to determine nitrate in plants, soils, and water. It is also used to produce adhesive/sealant and resins as negative photoresist for electroplating.
| | References | [1] R. R. Elton-Bott, A modified spectrophotometric method for nitrate in plants, soils and water by nitration of 3,4-dimethylphenol, Analytica Chimica Acta, 1977, vol. 90, 215-221
[2] George A. Burdock, Fenaroli’s Handbook of Flavor Ingredients, Sixth Edition, 2010
[3] Chia L. Wang, John Davis, Bryan Lienke, Polyurethane systems having non-sag paintability and primerless adhesion on concrete, Patent, 2012
[4] Yasuo Masuda, Yasushi Washio, Koji Saito, Method of forming plated product using negative photoresist composition and photosensitive composition used therein, Patent, 2012
| | Chemical Properties | YELLOW TO BROWNISH CRYSTALLINE MASS | | Occurrence | Reported found in coffee, tomato, parmesan and romano cheese, smoked fatty fish, white wine, katsuobushi
(dried bonito) and wood vinegar. | | Uses | Applications of 3,4-dimethylphenol:- It can react with ethyl cinnamates in trifluoroacetic acid to form the corresponding dihydrocoumarin derivatives.
- Bromination of 3,4-dimethylphenol using bromine can lead to 6-bromo-3,4-dimethylphenol.
- It can react with 1-fluoro-4-chloromethyl-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoro-borate) (Selectfluor? F-TEDA-BF4) to form 4-fluoro-3,4-dimethylcyclohexa-2,5-dienone.
| | Aroma threshold values | Detection: 1.2 ppm | | General Description | Colorless to light tan crystalline powder or solid. Odor threshold 1.2 mg/L. Taste threshold 0.05 mg/L. | | Air & Water Reactions | Hygroscopic. Insoluble in water. | | Reactivity Profile | 3,4-Dimethylphenol is incompatible with bases, acid chlorides, acid anhydrides, and oxidizing agents. 3,4-Dimethylphenol corrodes steel, brass, copper, and copper alloys. | | Fire Hazard | Flash point data for 3,4-Dimethylphenol are not available. 3,4-Dimethylphenol is probably combustible. | | Purification Methods | Heat 3,4-xylenol with an equal weight of conc H2SO4 at 103-105o for 2-3hours, then dilute it with four volumes of water, reflux it for 1hour, and either steam distil or extract it repeatedly with diethyl ether after cooling to room temperature. The steam distillate is also extracted and evaporated to dryness. (The purification process depends on the much slower sulfonation of 3,4-dimethylphenol than most of its likely contaminants.). It can also be crystallised from water, hexane or pet ether, and sublimed in a vacuum. [Kester Ind Eng Chem (Anal Ed) 24 770 1932, Bernasconi & Paschalis J Am Chem Soc 108 29691986, Beilstein 6 IV 3099.] |
| 3,4-Dimethylphenol Preparation Products And Raw materials |
|