Other grades of this product :
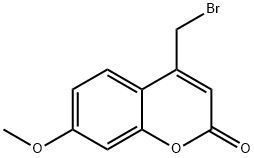
| 4-Bromomethyl-7-methoxycoumarin Basic information |
| 4-Bromomethyl-7-methoxycoumarin Chemical Properties |
| Melting point | 213-215 °C (lit.) | | Boiling point | 385.3±42.0 °C(Predicted) | | density | 1.552±0.06 g/cm3(Predicted) | | storage temp. | Keep in dark place,Inert atmosphere,2-8°C | | solubility | chloroform: soluble20mg/mL, clear, colorless to faintly yellow | | form | Powder and/or Chunks | | color | Off-white to light yellow or beige | | Water Solubility | insoluble | | λmax | 330 nm (MeOH) | | BRN | 187211 | | Stability: | Stable, but moisture and light-sensitive. Combustible. Incompatible with strong oxidizing agents. | | Major Application | Detection of nitrated
explosives; measuring ultraviolet ray power;
photoacid generators; polyurethane foams;
photoresists silica nanoparticles | | Biological Applications | Analyzing/detecting
carboxylic acids;analyzing/labeling fatty acids;
detecting nitric oxide; detecting/separating bile
acids; labeling pyrimidine nucleobases;
anticancer agents; biomedical trace analysis;
herbicides; as a substrate for measuring
histone acetyltransferases (HATs) activity, peptidases
activity, sirtuin activity | | CAS DataBase Reference | 35231-44-8(CAS DataBase Reference) |
| Hazard Codes | Xi,Xn | | Risk Statements | 36/37/38-22 | | Safety Statements | 26-36-37/39 | | RIDADR | 1759 | | WGK Germany | 3 | | HazardClass | 8 | | PackingGroup | III | | HS Code | 29322090 |
| 4-Bromomethyl-7-methoxycoumarin Usage And Synthesis |
| Chemical Properties | Light Green-Yellow Crystals | | Uses | 4-Bromomethyl-7-methoxycoumarin can be used as fluorescent label for fatty acids. For derivatization, TLC and HPLC separation, and fluorometric analysis of a wide range of naturally occurring acids, including bile and thromboxane B2.
| | Uses | Used for derivatization, TLC and HPLC separation, and fluorometric analysis of a wide range of naturally occurring acids, including bile and thromboxane B2 | | Uses | 4-Bromomethyl-7-methoxycoumarin has been used:- as derivatization reagent in determination of 5-fluorouracil (5-FU) in human plasma by HPLC-tandem mass spectrometry
- as derivatization reagent in fluorescence detection of sodium monofluoroacetate (MFA-Na) in biological samples by HPLC
- in the synthesis of new caged derivatives of hydrolysis-resistant 8-bromoadenosine cyclic 3′,5′-monophosphate (8-Br-cAMP) and 8-bromoguanosine cyclic 3′,5′-monophosphate (8-Br-cGMP)
- in TLC and HPLC separation and fluorometric analysis of a wide range of naturally occurring acids, including bile and thromboxane B2
| | Purification Methods | The coumarin crystallises from boiling AcOH; the crystals are washed with AcOH, EtOH and dried in a vacuum; 1HNMR (TFA) 3.97s, 4.57s, 6.62s, 6.92-7.19m and 7.80d. [Secrist et al. Biochem Biophys Res Commun 45 1262 1971, Dünges Anal Chem 49 442 1977, Beilstein 18 III/IV 348.] |
| 4-Bromomethyl-7-methoxycoumarin Preparation Products And Raw materials |
|