Other grades of this product :
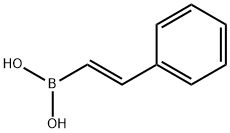
| E-PHENYLETHENYLBORONIC ACID Basic information |
| E-PHENYLETHENYLBORONIC ACID Chemical Properties |
| Melting point | 146-156 °C(lit.) | | Boiling point | 315.9±35.0 °C(Predicted) | | density | 1.130±0.06 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Store in freezer, under -20°C | | form | Powder and/or Chunks | | pka | 9.38±0.43(Predicted) | | color | Light yellow to beige | | CAS DataBase Reference | 6783-05-7(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 37/39-26 | | WGK Germany | 3 | | HazardClass | IRRITANT | | HS Code | 29310099 |
| E-PHENYLETHENYLBORONIC ACID Usage And Synthesis |
| Chemical Properties | Brown granular powder | | Uses | E-Phenylethenylboronic acid is a reagent used for• ;Palladium (Pd)-catalyzed Suzuki-Miyaura coupling reactions
1 Rhodium (Rh)-catalyzed intramolecular amination of aryl azides
2 Diastereoselective synthesis via Pd-catalyzed Heck-Suzuki cascade reaction
3 Copper (Cu)-mediated cyanation
4 Rhodium (Rh)-catalyzed asymmetric addition
5 Diastereoselective synthesis via iridium (Ir)-catalyzed addition
6 Palladium (Pd)-catalyzed cascade cyclization
7 Reagent used in Preparation of • ;Optically active unsaturated amino acids by diastereoselective Petasis borono-Mannich reactio
| | Uses | trans-beta-Styrylboronic acid as reagent is used for palladium (Pd)-catalyzed Suzuki-Miyaura coupling reactions, diastereoselective synthesis via Pd-catalyzed Heck-Suzuki cascade reaction and rhodium (Rh)-catalyzed intramolecular amination of aryl azides. It is also used as reagent in preparation of optically active unsaturated amino acids by diastereoselective Petasis borono-Mannich reaction and amino alcohol dienes via Petasis 3-component reaction using Ru-catalyzed ring-closing metathesis and isomerization. | | Uses | Reagent used for- Palladium (Pd)-catalyzed Suzuki-Miyaura coupling reactions
- Rhodium (Rh)-catalyzed intramolecular amination of aryl azides
- Diastereoselective synthesis via Pd-catalyzed Heck-Suzuki cascade reaction
- Copper (Cu)-mediated cyanation
- Rhodium (Rh)-catalyzed asymmetric addition
- Diastereoselective synthesis via iridium (Ir)-catalyzed addition
- Palladium (Pd)-catalyzed cascade cyclization
Reagent used in Preparation of - Optically active unsaturated amino acids by diastereoselective Petasis borono-Mannich reaction
- Amino alcohol dienes via Petasis 3-component reaction using Ru-catalyzed ring-closing metathesis and isomerization
|
| E-PHENYLETHENYLBORONIC ACID Preparation Products And Raw materials |
| Raw materials | Phenylacetylene-->CATECHOLBORANE-->1,3,2-Dioxaborolane, 2,2',2''-methylidynetris--->POTASSIUM BETA-STYRYLTRIFLUOROBORATE-->[(Z)-2-bromoethenyl]benzene-->TRANS-2-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)STYRENE-->2,2'-Bis-1,3,2-benzodioxaborole-->Benzaldehyde-->Water | | Preparation Products | trans,trans-Dibenzylideneacetone-->(E)-2-Nitroethenylbenzene |
|